To become the leading pharmaceutical company
Dongying East pharmaceutical company is the largest producer of enoxaparin sodium, is also China's first world third manufacturers received enoxaparin COS certificate
- 280
No. 280 in Top 500 Chinese Enterprises
- 138
No. 138 in Top 500 Chinese Manufacturing Enterprises
- 1
No. 1 in Top 100 Chinese Special Chemical Manufacturing Enterprises
Tiandong company profile
Dongying Tian Dong pharmaceutical co was established in 1992, under the Shandong Ocean chemical group, specializing in viscous polysaccharide product development, production and sales, in Shandong province is "high-tech enterprise".






Our products
Tiandong company has a good quality management system, have passed the EU COS certification, EDQM site audit and United States FDA site audit, access to CEP certificates, Sweden GMP certificates, Brazil ANVISA GMP certificates, France GMP certificate, completed a number of countries and regions registered products are sold in more than 40 countries and regions.
- API Series: Heparin Sodium
- Product Qualification: GMP (China), India registration No. BD-711
- Product Standard: EP/BP/USP/IP/CP
- Product Effects: Some of its ingredients work well in extending blood clotting time.
- API Series: Nadroparin Calcium
- Product Standard: EP
- Product Effects: Anticoagulants, preferred choice for deep venous thrombosi
- API Series: Enoxaparin Sodium
- Product Qualification: COS, GMP (France), GMP (Sweden), GMP (Brazil)
- Product Standard: EP/BP/USP/IP
- embolism; Treat deep venous thrombosis; Prevent the formation of thrombus in
- extracorporeal circulation during hemodialysis, and treat unstable angina.
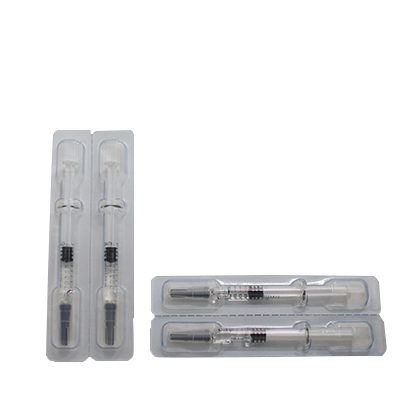
- Preparations: Enoxaparin Preparation
- Preparations: Heparin Sodium Preparation
- Preparations: Dalteparin Sodium Preparation
- Preparations: Nadroparin Calcium Preparation
- API Series: Dalteparin sodium
- Product Standard: EP
- Product Effects: Prevention and treatment of thrombosis formation and embolism,
- treatment of disseminated intravascular coagulation of various causes.
Marketing service network
The company's products are exported to Germany (FK), India, Belarus, South Korea, Poland, Chile, Peru, Switzerland, Tunisia, Moldova, Guatemala, America (Mylan), Argentina, Russia, Turkey, Cuba, Israel (Teva), India, Brazil, Bangladesh, Columbia, Germany, Switzerland, Tunisia, Turkey, Ireland Iran and other more than and 40 countries and regions.

haike gROUP
Industry advantage
-

Largest low molecular weight heparin producer
-

Innovative research and development
-
Dongying days East Pharmaceutical Co., Ltd. since 2010, a total of 15 patents for invention patents, utility model patents 8, a total of 23.
One, the invention patent 15
1, removal of oversulfated chondroitin sulfate in heparin sodium
2, heparin sulfate in the quantitative detection method of skin
3, a method for production of heparin
4. A method for the separation of an ester
5, a method of production of heparin
Method for direct production of enoxaparin 6 a kind of coarse heparin sodium
7, a low molecular weight chitosan and amino glucose co production process
8, a process using chondroitinase production of heparan sulfate
9, a kind of heparin sodium preparation technology
10, high efficiency heparin sodium raw material refining process
11, a low molecular weight heparin production of free sulfate ion rapid detection method
12. A process for extracting sulfuric acid from the duodenum.
13, a chelating resin for the removal of copper ion
14, a method for rapid separation of fast moving heparin and sulfate
15. An analytical method for the identification of oligosaccharides from low molecular weight heparin using high performance liquid chromatography.
Two, practical patent 8
1, the laboratory is easy to clean the mixer
2, heparin titer detector
3, new type optical rotation instrument temperature control device
4, new chemical experimental ventilation cabinet
5, a new type of high efficiency ultrasonic tank
6, an online detection of alcohol concentration of heparin reaction tank
7, heparin sodium production device
8, an improved ultrasonic low-temperature extraction tank
-
-

Through international certification
-
The day of the east company has good production quality management system, has adopted a series of international certification regulatory departments.
In 2004, the production license of heparin sodium.
In 2006, the enoxaparin drug production license
On 2007, the day of the east company enoxaparin sodium through the EU COS certification, is the only domestic ENOXAPRIAN products through the EU COS certification.
In 2007 and 2009, through the on-site audit of enoxaparin EDQM
In 2008 and 2010, received enoxaparin CEP certificate
In 2008, the Swedish enoxaparin GMP certificate
In 2009, the Brazil ANVISA GMP certificate of enoxaparin sodium
In 2010, the French ENOXAPRIAN GMP certificate
On August 2013, the day of the east company of heparin and enoxaparin sodium two drug varieties passed the United States FDA on-site audit.
-
-

Products are exported to more than and 40 countries and regions
- The company has also made ENOXAPRIAN in Brazil, Sweden and France's GMP certificate, and the completion of a number of countries and regions of the registration, the products are exported to Germany (FK), India, Belarus, South Korea, Poland, Chile, Peru, Switzerland, Tunisia, Moldova, Guatemala, Argentina, United States (Mylan) Turkey, Cuba, Russia, Israel, India, (Teva), Brazil, Bangladesh, Columbia, Germany, Switzerland, Tunisia, Turkey, Ireland, Iran and other more than and 40 countries and regions.
-

Participate in the formulation of 5 kinds of low molecular weight heparin quality standards
Haike subsidiary engaged in pharmaceutical field
Dongying Tian Dong Pharmaceutical Co., Ltd.
Enter the subsidiary website>>The largest low molecular weight heparin producer in China
Enter the subsidiary website>>-

The official WeChat Haike
National Service Hotline
0546-7080213 0546-7791088Fax:0546-8180302
Email:market@td-pharm.com